Biomarkers in the Context of Epilepsy
Epilepsy is one of the most common neurological disorders, accounting for about 1% of the global burden of diseases based on premature deaths or years lost because of a disability (Dixit, Tripathi, Chandra, & Banerjee, 2016). Currently, it is estimated that 2.4 million people globally are diagnosed with epilepsy yearly. In about 60% of cases, epileptogenesis occurs because of structural causes, including stroke or traumatic brain injury (TBI). Several hypothesis-based monotherapy methods have shown some disease-modifying impacts in animal models of epileptogenesis. However, no clinical treatment is currently available to alleviate or stop the cause of epilepsy after diagnosis or stop epileptogenesis in the at-risk patient, and the development towards epilepsy treatment remains a baffling medical need among researchers (Scheffer et al. 2017). Both cellular and molecular biomarkers and electrical and imaging examinations provide a specific platform for describing epileptogenic zone (EZ). Most researchers face the primary challenge of finding biomarkers that can accurately localize epileptogenic zone to complement electrical and imaging examinations currently adopted. Biomarkers are any measurement that reflects the interaction between biological systems and potential hazards, physical, chemical, or biological (Louro et al., 2019). In this paper, the researcher explores the present findings on molecular epilepsy biomarkers.

- FAST HOMEWORK HELP
- HELP FROM TOP TUTORS
- ZERO PLAGIARISM
- NO AI USED
- SECURE PAYMENT SYSTEM
- PRIVACY GUARANTEED
Biomarkers of epilepsy combine factor specific and sensitive and can be objectively be identified and inferred as indicators of pathological changes associated with epileptogenesis. At the molecular levels, epileptogenesis is the process where early brain-damaging insult activates a cascade of molecular changes, ultimately leading to the incidences of unprovoked seizures, as shown in figure 1.
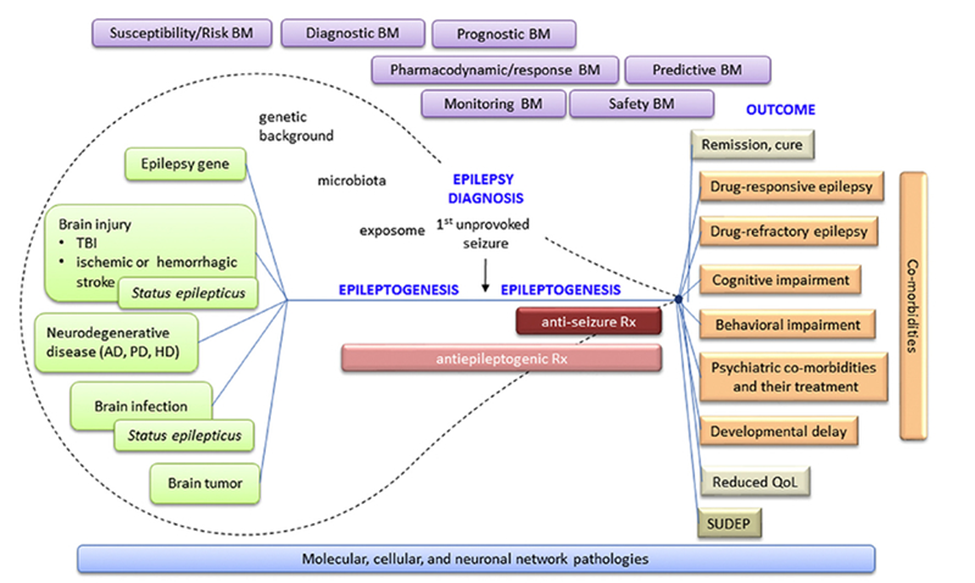
Figure 1. Epileptogenic Process. Epileptogenesis is started with the “epilepsy gene” or different types of chronic neurodegenerative diseases or acute brain insults (Pitkänen, 2018).
Epileptogenesis following a transitory insult to the brain system is complemented by a pathogenic process, often serving as a potential source for epilepsy biomarkers. The processes comprise reactive astrogliosis, blood-brain barrier (BBB) dysfunctions, the existence of activated leukocytes and microglia cells, neurogenesis axonal regeneration, neuronal cell loss, and distribution of ion channels and neurotransmitter receptors (Auvin et al. 2017). Targeting the channels and receptors may be mainly suited to adjusting the epileptic threshold (Dixit, Tripathi, Chandra, & Banerjee, 2016). Focal epilepsies provide a more encouraging environment for the molecular biomarkers’ development than other central nervous disorders, lacking native disease tissues. Study shows that many patients diagnosed with focal epilepsies often exhibit pharmacoresistant to antiepileptic medicines (Simonato et al., 2021). As a result, surgical treatment for epileptogenic centers on seizure control outcomes.
The complementary presence of brain tissues from focal epilepsy patients under surgery or undergoing peripheral blood and cerebrospinal fluid (CSF) from various time points corresponding to the time point for the neurosurgical interventions is valuable in discovering biomarkers. If the CSF and blood replicate pathogenic mechanisms in epileptogenic brain tissues, the patient is most appropriate for detecting molecular biomarkers (Engel & Pitkänen, 2020). The complementary method may comprise the assessment and characterization of possible biomarkers in animal representations of focal epilepsy and their likely translation to the human patient, as shown in figure 2.
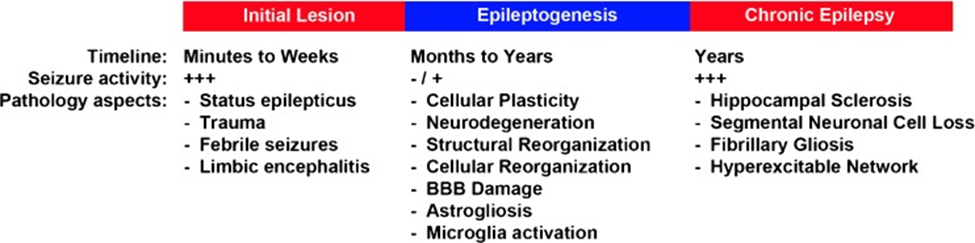
Figure 2.The schematic representation of crucial changes during epileptogenesis. A potential biomarker and plethora of mechanisms contribute to the changing from a normal to an acute epileptic brain structure (Lukasiuk & Becker, 2014). The development of biomarkers in epilepsy is noticeably a more complex process.

ORDER A CUSTOM ESSAY NOW
HIRE ESSAY TYPERS AND ENJOT EXCELLENT GRADES
Researchers have proposed two significant differences in the developing biomarkers. The first process concerns possible epileptogenesis following a temporary insult to the brain. In such a case, the recognized/putative molecular biomarkers must envisage the future advancement of a disease that has not emerged at the time of the biomarkers evaluation. The second aspect, which represents a significant deviation from neuro-oncology molecular biomarker for epilepsy, reflects the intricate seizure propensity dynamics (Rinschen, Ivanisevic, Giera & Siuzdak, 2019). They may also include the surrogate markers demonstrating the potential to retard or pharmacologically antagonize epileptogenesis, hence avoiding any epilepsy-related surgery for the patient (Klein & Tyrlikova, 2017). Such considerations demonstrate that even though brain tissues from patients under epilepsy surgical treatment in the pharmaco-resistant phase can be adopted for molecular biomarker development, such molecules may be predominantly beneficial if they can be examined in the blood or cerebral spinal fluid (CSF) (Walker, Janigro, Heinemann, Riikonen, Bernard, & Patel, 2016).
Collection of CSF, blood, or brain tissue can offer vital information at molecular levels. High throughput approaches, including proteomics, genomics, and metabolomics, allow surveillance of several diverse molecular entities in just a few biological samples, enabling researchers to hone in on particular biomarkers often expressed in an epilepsy disease condition (Rinschen, Ivanisevic, Giera & Siuzdak, 2019). Such biomarkers can be associated with many imaging and electrophysiological biomarkers highlighted in figure 3.
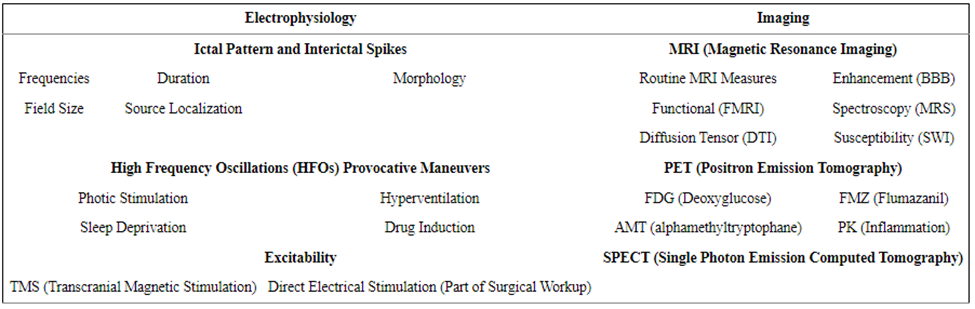
Figure 3. Imaging and electrophysiological epilepsy biomarkers (Engel & Pitkänen, 2020).
In the case of CSF or blood tests, one can follow a particular biomarker as a function of time because it is associated with the disease state. On the one hand, brain tissue sampling can only be done once and offers information at a particular point in time. Because a patient with pathologically refractory epilepsy undertakes surgical resections of the epileptogenic regions, epilepsy is among the few brain disorders with fresh human brain tissues, highly characterized for studies (Raoof et al., 2017). The only challenge is that the disease is often already established when the brain tissue is removed. However, in the animal model of epilepsy, the brain tissue can be harvested from a particular epileptic model based on different time points, permitting epileptogenesis examination at multiple stages (Avansini et al., 2017). Besides the homogenizing tissues and examining them for metabolic levels, protein, and ribonucleic acid (RNA), spatially controlled markers of different pathological progressions revealed by chronological staining may also serve as biomarkers of diverse disease conditions. Such processes have been described and entail alterations in blood-brain barriers, loss of neurons, inflammation, gliosis, angiogenesis, axonal sprouting, neurogenesis, and synaptic reorganization. Also, chronic and acute samples of slices taken from human epileptic brain zones along with animal models are a way to functionally pinpoint specific measures of network changes and excitability ((Rinschen, Ivanisevic, Giera & Siuzdak, 2019).
Biomarkers (circulating biologic markers) in epilepsy have several potential uses, such as the potential to predict epilepsy development after a brain insult resulting from the first seizure, enhance pharmacovigilance by recognizing vulnerability to adverse drug reactions (ADRs), and prognosticating disease development and pharmacoresistant to antiepileptic drugs subsequent to the first diagnosis. Biomarkers are essential to the whole process of drug development, from preclinical safety indications in the early stages of drug development trials in a smaller sample of the population to assessing a larger population for safety indications (Pescini et al., 2017), as shown in figure 3. Biomarkers of epilepsy development or (epileptogenesis) and the propensity to produce spontaneous seizure (ictogenesis) can also emerge from epilepsy conditions, establish the presence and severity of a tissue that can generate spontaneous seizure, and assess the epilepsy condition once established. It can also be applied to develop animal models to screen the potential antiseizure drugs and antiepileptogenic and devices cost-effectively while also minimizing the cost of medical trials of possible antiepileptogenic interventions as it enriches the trial population with patients at high risk of epilepsy development and serving as surrogate markers for spontaneous behavioral seizures (Pitkänen et al. 2016).
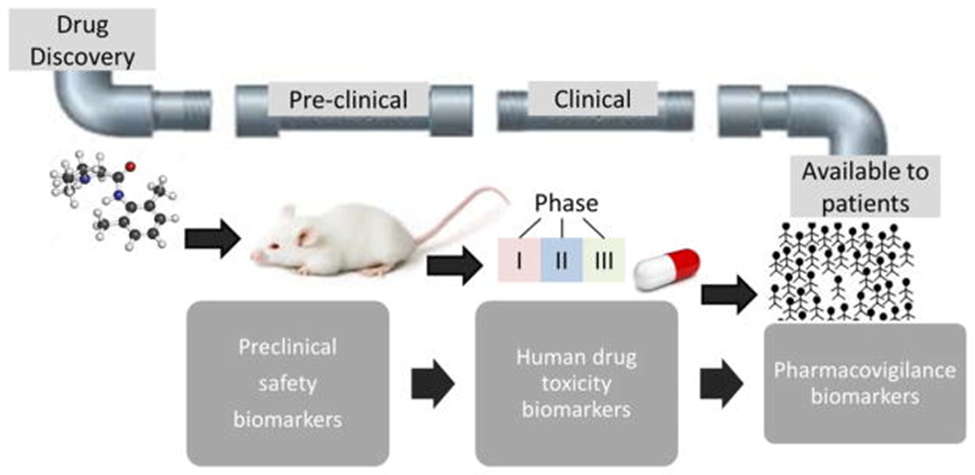
Figure 4. Biomarkers in the developmental pipeline for new drugs (Walker et al. 2016).
The biomarkers of epileptogenesis are, however, challenging and overpriced to discover. Only a few people will develop epilepsy after severe epileptogenic brain insult, including penetrating head injuries. Besides, the process may take more than 10 years (Vitaliti, Pavone, Marino, Saporito, Corsello, & Falsaperla, 2019). That explains why few studies are done on preventing seizure disorders following a brain insult.
Overall, this study explored findings on molecular epilepsy biomarkers. The study reveals that biomarker development is a complex process, which explains why the field of epilepsy currently still lacks or faces slow development of biomarkers that can reliably establish near or at diagnosis patient’s potential for developing drug-resistant epilepsy. Researchers are still facing the challenge of finding biomarkers that can accurately localize epileptogenic zone to complement electrical and imaging examinations currently adopted. Currently, no clinical treatment is available to alleviate or stop the cause of epilepsy after diagnosis or stop epileptogenesis in the at-risk patient. However, the development towards epilepsy treatment remains a baffling medical need among researchers, with several trials including disease-modifying impacts in animal models of epileptogenesis, which may soon yield a positive result.
References
Auvin, S., Walker, L., Gallentine, W., Jozwiak, S., Tombini, M., & Sills, G. J. (2017). Prospective clinical trials to investigate clinical and molecular biomarkers. Epilepsia, 58, 20-26.
Avansini, S. H., de Sousa Lima, B. P., Secolin, R., Santos, M. L., Coan, A. C., Vieira, A. S., … & Lopes-Cendes, I. (2017). MicroRNA hsa-miR-134 is a circulating biomarker for mesial temporal lobe epilepsy. PLoS One, 12(4), e0173060.

